Registrar Corp., Hampton, Va., updated the FDA Compliance Monitor with a new simplified layout and optimized backend drive user efficiency.
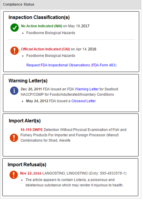
The FDA Compliance Monitor assists companies with supply chain monitoring requirements under the U.S. Food and Drug Administration (FDA) Preventive Controls and Foreign Supplier Verification Program (FSVP) rules. It aggregates and supplements data from five FDA databases to supply comprehensive, up-to-date compliance reports on all FDA-regulated companies. The monitor also automatically sends users email alerts whenever the published FDA compliance status of a supplier changes.
On the front end, users now see a series of compact rows that display an overview of supplier compliance; these rows can be further expanded to review detailed company data for each supplier.
The next set of rollouts will include:
- Validation of FDA registration numbers.
- A repository for requesting and storing HACCP and HARPC food safety plans as well as other documentation for supplier compliance.
- Enterprise integration capabilities.
Registrar Corp.
757-224-0177
www.registrarcorp.com