The year 2022 marks a significant impasse for a variety of food safety-related regulations on the American Frozen Food Institute’s (AFFI) public policy agenda that should also be top of mind for your company.
Listeria
This summer, the U.S. Food and Drug Administration’s (FDA) Center for Food Safety and Applied Nutrition (CFSAN) and Office of Food Policy and Response (OFPR) released a list of draft and final guidance topics that the agency intends to publish over a 12-month timeframe. That lists includes a compliance policy guide (CPG) for Listeria monocytogenes (Lm). AFFI expects the CPG will define FDA’s regulatory approach for Listeria and whether an action level greater than zero will be acceptable for ready-to-eat (RTE) foods that do not support the growth of Lm.
The FDA similarly indicated publication of guidance to classify food as RTE or not-ready-to-eat (NRTE). Without clear definitions, status of a food can be assessed subjectively by the FDA’s Office of Regulatory Affairs.
As part of AFFI’s journey to advance food safety, we have advocated for foods to be considered NRTE if they are clearly labeled with cooking instructions validated to serve as a final kill step. The FDA has been leaning toward declaring foods RTE if consumers expect the food to be RTE (and have used frozen peas and chicken with grill marks as examples).
This year marked an important milestone in the journey to achieve science-based and practical alternatives to current approach to target Lm. AFFI’s inaugural Food Safety Forum garnered more than 700 registrants comprised of global research experts, industry professionals from across the broader food industry and food safety policy leaders.
As stated by former FDA CFSAN Director, Dr. Robert Brackett, in an article reflecting on the outcomes of AFFI’s Food Safety Forum, “It’s time for the food industry and regulators to be bold and try something new.” While AFFI recognizes that current hazard-based regulatory and enforcement policies do not engender an easy transition to a more risk-based monitoring program, grounded with the latest science, we continue to stress the importance of using risk-based approaches to regulate the presence of Lm across the broad category of low-risk foods with both FDA and the U.S. Department of Agriculture (USDA) Food Safety Inspection Service (the two federal agencies that largely regulate the presence of Lm).
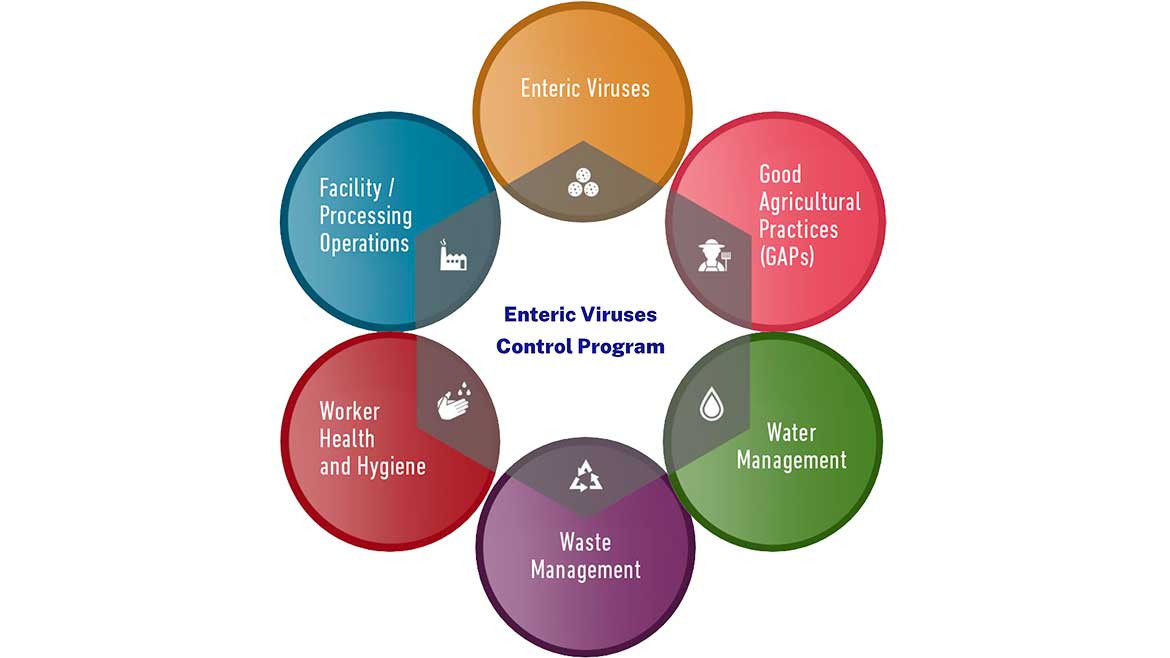
Enteric Viruses (EV)
U.S. imports of frozen and fresh fruits rose by 11% in value from September 2020 through August 2021 with imports of berries growing by 23%. As U.S. demand for imported frozen berries increases, it is critical for all entities across the supply chain to ensure the highest food safety standards are applied from growing to processing and packing.
The frozen berry supply chain is a global network of producers and processors with disparate manufacturing practices and conditions. It is also well documented that several regions that are important sources of frozen berries are endemic for Hepatitis A and norovirus infections thus increasing the risk of contamination in these growing and processing areas. That’s why this year, AFFI expanded its Food Safety Zone to include an Enteric Viruses Control Program (EVCP). These best practices were put together by food safety professionals from across the food and agriculture sector and consolidates a variety of technical materials and templates developed by experts in academia and industry. AFFI strongly believe these steps will elevate the industry’s ability to safety produce and process frozen berries.
How does this tie back to regulation? In 2019, the FDA undertook a frozen berry surveillance program to assess the prevalence of enteric viruses in both domestic and imported produce. While the agency’s plan was not rooted in previous outbreaks or known systemic risks associated with the domestic supply, AFFI supported the initiative with the prospect of increasing scientific data related to these products. It resulted in several unsubstantiated frozen berry recalls through the Summer of 2019, that led to media reports calling in to question the safety of frozen berries. It’s important to note that since the beginning of the agency’s surveillance effort there has not been a single illness associated with enteric viruses in frozen berries and as COVID-19 crossed into U.S. shores, the agency temporarily-suspended the program in March 2020.

Supply Chain Demands
The COVID-19 pandemic placed unprecedented stresses on food supply chains, with bottlenecks in farm labor, processing, transportation and logistics, as well as significant shifts in demand for inputs, such as packaging, ingredients, and sanitation and PPE supplies. In response to COVID-19, regulatory flexibility was allowed by both the FDA and USDA, such as labeling compliance flexibility due to ingredient and product shortages. AFFI has joined with our partner food and beverage trade associations in calling on the Biden administration to extend this regulatory flexibility until the pandemic recedes and the supply chain disruptions are resolved.
New Era Of Smarter Food Safety And FDA Traceability Rule
The Food Traceability Proposed Rule is a key component of FDA’s New Era of Smarter Food Safety Blueprint and would implement Section 204(d) of the Food Safety Modernization Act (FSMA). If finalized in its current form in 2022, this rule would establish a standardized approach to traceability recordkeeping, paving the way for industry to adopt, harmonize, and leverage more digital traceability systems in the future, according to the FDA. While frozen foods are not explicitly included in the list of high-risk foods, there’s a good chance many foods—especially frozen produce—will have to comply and that the rule will influence practices throughout the supply chain, even for foods not identified by the FDA as high risk.
To allow the FDA the ability to rapidly track and trace food, the proposed rule will mandate that companies use a traceability lot code and require maintaining records throughout the supply chain linking the traceability lot code to additional information, such as where it’s grown, who shipped it, receiving and shipping information, and more. This goes beyond the one up, one back traceability models that are currently in place.
However, that is easier said than done. AFFI recently led a series of traceability workshops where several categories of foods presented case studies about how current tracking and tracing approaches can meet the requirements of the FDA’s Traceability Rule with combinations of paper and digital systems.
Grounded in science, AFFI will continue to advocate for practical food safety regulations on behalf of the frozen food industry. In turn, I encourage you to visit AFFI’s Food Safety Zone, share it with others in your company and begin implementing the variety of food safety resources and programs we offer.




